The material of the ceramic metal halide arc tube is polycrystalline alumina (PCA). Below 1150 °C, PCA generally does not chemically react with the filled metal halide, so the working temperature of the ceramic arc tube is higher than that of the quartz arc tube. 200 ° C, which makes the ceramic metal halide lamp's light efficiency and color rendering further improved.
Although ceramic arc tubes can withstand higher temperatures, the development of ceramic metal halide lamps is still limited. Among them, in the case of relatively high temperature, the inner wall material of the ceramic tube is chemically reacted with the metal halide to be corroded [1], causing the temperature of the tube wall to change, resulting in a change in the color and electric parameters of the lamp. Especially in the case of materials and processes are not mature, severe corrosion will cause the ceramic tube wall to be white, affecting the light, and even causing the arc tube to leak and the lamp to fail. Therefore, studying the corrosion mechanism of the tube wall in ceramic metal halide lamps and various factors affecting the degree of corrosion is of great significance for optimizing the lamp making process, extending the lamp life and improving the lamp characteristics.
1 Temperature distribution and convection in the arc tube
Under working conditions, the arc temperature of the ceramic metal halide lamp is much higher than the wall temperature, so the temperature gradient inside the arc tube is very large, which will generate strong convection, flow upward in the middle of the arc tube, and flow downward at the tube wall. . Figure 1 shows the temperature distribution inside a ceramic arc tube under working conditions. The temperature of the shaft is the highest, up to 6000 K; the temperature at the arc attachment point on the electrode is about 3000 K; the temperature of the tube wall is the lowest, only 1300 K. The strong convection in the arc tube determines the position of the cold end. Hilpert et al. describe the temperature distribution in a vertical arc ceramic arc tube [2]. As shown in Figure 1 (a), the arc is on the central axis, with the highest temperature near the lower electrode and the cold end near the lower electrode. Van Erk et al. describe a temperature distribution in an arc tube with a horizontal ignition point [3]. As shown in Figure 1 (b), convection within the tube causes the arc to float, making the temperature distribution of the arc tube asymmetrical, with the upper end close to the arc and the highest temperature (1550). K) ; The lower end is away from the arc and the temperature is the lowest (1300 K), which is the cold end.
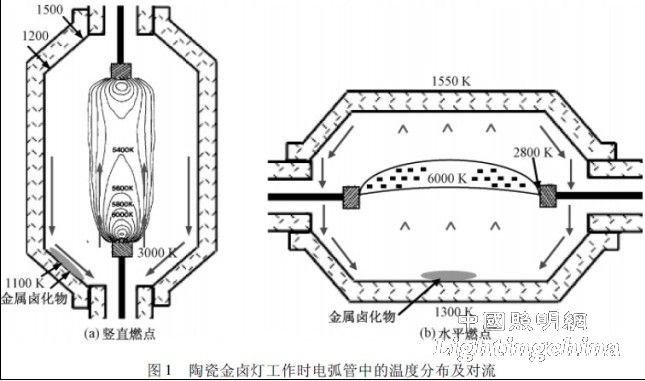
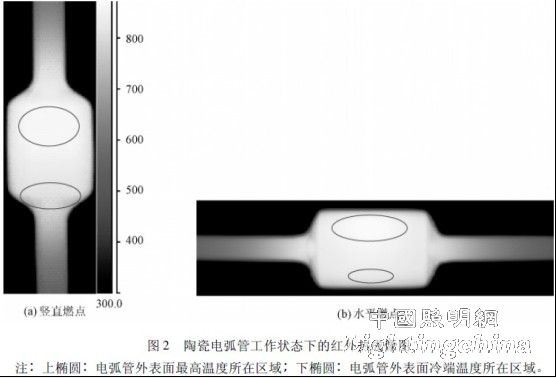
Figure 2 is an infrared thermal imaging diagram of the measured state of the 70 W ceramic arc tube. Influenced by the convection in the arc tube at the two ignition positions, the highest temperature and the cold end temperature of the outer surface of the arc tube are in the upper ellipse and the lower elliptical region, respectively.
The convection in the arc tube at the ignition point of the lamp determines the corrosion of the inner wall of the arc tube. On the one hand, the gas phase metal halides are transported by convection, in the high temperature zone of the pipe wall and the pipe wall material. ![]() Reaction, corroding the tube wall. The product of the reaction is transported in the arc tube with convection. On the other hand, convection determines the cold end of the inner wall of the arc tube, and excess metal halide is deposited as a molten salt on the cold end to corrode the cold end of the tube wall.
Reaction, corroding the tube wall. The product of the reaction is transported in the arc tube with convection. On the other hand, convection determines the cold end of the inner wall of the arc tube, and excess metal halide is deposited as a molten salt on the cold end to corrode the cold end of the tube wall.
2 Corrosion mechanism of the pipe wall
The arc tube is usually filled with an excess of metal halide pellets which partially evaporate at high temperatures in the tube, partially in the form of molten salts, and reach a saturated vapor pressure at equilibrium. The corrosion of the inner wall of the ceramic tube is mainly caused by two factors: First, the gas phase metal halide salt and the wall material ![]() The corrosion caused by the reaction; the second is the deposition of metal halide molten salt on the cold end, corrosion of the cold end pipe wall [3].
The corrosion caused by the reaction; the second is the deposition of metal halide molten salt on the cold end, corrosion of the cold end pipe wall [3].
Take ![]() For example, the following chemical reactions occur mainly in the corrosion of the pipe wall by gas phase metal halide salts [3]:
For example, the following chemical reactions occur mainly in the corrosion of the pipe wall by gas phase metal halide salts [3]:
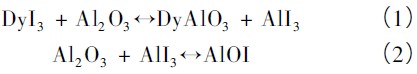
Metal halide salt in reaction (1) ![]() Wall material
Wall material ![]() Reaction, generation
Reaction, generation ![]() Further reacting with the wall material to form AlOI. Where the product in reaction (1)
Further reacting with the wall material to form AlOI. Where the product in reaction (1) ![]() As a transport agent, the reaction is carried out at a high temperature (2) to the right, and at a low temperature (2) to the left. Therefore, in the gas phase material,
As a transport agent, the reaction is carried out at a high temperature (2) to the right, and at a low temperature (2) to the left. Therefore, in the gas phase material, ![]() It is transported to the low temperature zone by intense convection in the lamp, and AlI3 can be continuously replenished from the reaction (1) to continuously transport Al2O3 from the high temperature zone to the low temperature zone.
It is transported to the low temperature zone by intense convection in the lamp, and AlI3 can be continuously replenished from the reaction (1) to continuously transport Al2O3 from the high temperature zone to the low temperature zone.
In addition to the corrosion of the gas phase by the ceramic tube wall, the metal halide molten salt also corrodes the tube wall. But the mechanism of this corrosion is still unclear. Van Erk pointed out that the corrosion of the metal halide molten salt on the quartz tube wall is due to the molten salt. ![]() A phase change has occurred, changing from a glass phase to a crystal phase [3]. The corrosion mechanism of molten salt on PCA tube is still inconclusive, but it is not related to the structural change of PCA. Usually molten salt is deposited on the cold end of the inner tube wall, and severe corrosion of the tube wall by the molten salt may cause the tube wall to become whitish or even leak.
A phase change has occurred, changing from a glass phase to a crystal phase [3]. The corrosion mechanism of molten salt on PCA tube is still inconclusive, but it is not related to the structural change of PCA. Usually molten salt is deposited on the cold end of the inner tube wall, and severe corrosion of the tube wall by the molten salt may cause the tube wall to become whitish or even leak.
3 Transportation process in ceramic metal halide lamps
Treating the interior of an arc tube as a thermodynamic system, the process of transitioning the system from a non-equilibrium state to a balanced state is called a transport process. When the ceramic metal halide lamp is working, there are complex transportation processes in the arc tube. There are two main processes: chemical reaction and heat conduction. The above only describes the basic mechanism of corrosion of ceramic arc tube wall. In fact, anything related to the wall material ![]() The transport process is related to the corrosion of the pipe wall. To study the corrosion of the ceramic metal halide tube wall, it is necessary to study the transport process in the arc tube.
The transport process is related to the corrosion of the pipe wall. To study the corrosion of the ceramic metal halide tube wall, it is necessary to study the transport process in the arc tube.
The transport process in the arc tube is very complicated. Therefore, the main factors in the transport process are grasped, and the process is simplified. It is helpful to simulate and calculate the process, so as to make a qualitative and semi-quantitative analysis of the actual process. Fischer et al. simulated the transport process in the arc tube through a simplified model [4]. They simulated ![]() The system uses three reactions to describe the transport process within the tube. One of the reactions is shown in equation (2), and the other two reactions are related to the evaporation of Al2O3:
The system uses three reactions to describe the transport process within the tube. One of the reactions is shown in equation (2), and the other two reactions are related to the evaporation of Al2O3:
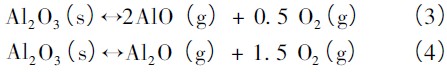
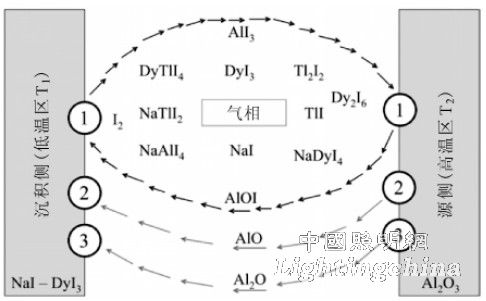
Figure 3 shows the transport process associated with the reaction (2 to 4) in the lamp. As mentioned above, reaction (2) is a reversible reaction associated with corrosion of the ceramic tube wall. In the high temperature zone, the reaction ( 2) is carried out to the right, the wall material ![]() Transported to the low temperature zone with AlOI as the carrier; in the low temperature zone, the reaction (2) proceeds to the left,
Transported to the low temperature zone with AlOI as the carrier; in the low temperature zone, the reaction (2) proceeds to the left, ![]() It is deposited in the low temperature zone while releasing AlI3 and continues to participate in the reaction in the high temperature zone. In this reversible reaction,
It is deposited in the low temperature zone while releasing AlI3 and continues to participate in the reaction in the high temperature zone. In this reversible reaction, ![]() Transported from the high temperature zone to the low temperature zone in the form of AlOI,
Transported from the high temperature zone to the low temperature zone in the form of AlOI, ![]() It is a transport agent that does not reduce itself, but maintains the response. Reaction (3,4) is
It is a transport agent that does not reduce itself, but maintains the response. Reaction (3,4) is ![]() Evaporation process, by evaporation, the wall material is AlO and
Evaporation process, by evaporation, the wall material is AlO and ![]() The form is transported to the low temperature zone where it condenses into
The form is transported to the low temperature zone where it condenses into ![]() Deposition. The simulation calculation by this model shows that when the temperature is lower than 1773 K, the transport process determined by the reaction (2) plays a leading role; when the temperature is higher than 1773 K,
Deposition. The simulation calculation by this model shows that when the temperature is lower than 1773 K, the transport process determined by the reaction (2) plays a leading role; when the temperature is higher than 1773 K, ![]() The evaporation (reaction (3,4)) determines the transport process dominates. In addition, the composition of the molten salt determines the composition of the phase material, which affects the transport process within the arc tube, which in turn affects the degree of corrosion. Another factor affecting the degree of corrosion is the temperature difference between the high temperature zone and the low temperature zone.
The evaporation (reaction (3,4)) determines the transport process dominates. In addition, the composition of the molten salt determines the composition of the phase material, which affects the transport process within the arc tube, which in turn affects the degree of corrosion. Another factor affecting the degree of corrosion is the temperature difference between the high temperature zone and the low temperature zone.